
China Largest Factory Manufacturer Supply Beta-Nicotinamide Mononucleotide(NMN) Cas 1094-61-7 For stock delivery
-
Purity99.9%
-
UseHealth Care
-
OriginChina
-
Package1KG/Tin 25KG/Drum*Carton
-
ManufacturerXI'AN LEADER BIOCHEMICAL ENGINEERING CO.,LTD
-
Place of OriginCHINA
-
Brand NameLeader
-
CertificationISO,GMP,SGS,HALA,KOSER,HACCP
-
Model NumberLD
-
Minimum Order Quantity25KGS
-
PriceNegotiate
-
Packaging Details25KG/Drum
-
Delivery Time2-3 working days
-
Payment TermsWestern Union, MoneyGram, T/T, L/C
-
Supply Ability10MTS/Month
China Largest Factory Manufacturer Supply Beta-Nicotinamide Mononucleotide(NMN) Cas 1094-61-7 For stock delivery
China Largest Factory Manufacturer Supply Beta-Nicotinamide Mononucleotide(NMN) Cas 1094-61-7 For stock deliveryβ-Nicotinamide mononucleotide (NMN) is a natural product which exists in small quantity in most plants, such as edamame, broccoli, and cucumber [1]. It is also an endogenous molecule in all mammalian tissues [2, 3]. Its biological functions are linked to the ability to boost nicotinamide adenine dinucleotide (NAD), a product of the salvage pathway in NAD biosynthesis in which NMN serves as a precursor [2, 3]. NAD concentration declines with age affecting mammalian longevity and age-related health conditions through its functions of energy metabolization and activations of poly ADP-ribose polymerase (PARP), Sirtuin proteins, and etc. in mammalian tissues [2, 4].Many preclinical studies on NMN supplementation have been reported [2–4]. NMN treatment of Werner syndrome (WS) worms and flies with 1mM dosing elevated NAD concentrations 2.2 times higher than control and extended lifespan to 19.8 days vs. 13.9 days in control [5]. NMN intervention to healthy wild-type 3-4-month C57BL/6N mice steadily increased liver NAD concentrations over 30 min after a single oral 300 mg/kg dose, and effectively mitigated age-associated physical decline and was well tolerated after a 12-month oral treatment of 100 mg/kg/day or 300 mg/kg/day [1]. Clinical benefits of NMN supplementation in mouse models have shown doubled running endurance on aging mice [6], restored cognition in Alzheimer’s disease rat model [7], and reversed vascular dysfunction in old mice [8]. However, human clinical trials with NMN are limited [9–17]. The first human clinical trial confirmed that NMN supplementation was safe up to 500 mg/day and plasma concentrations of NAD metabolites were significantly increased, but the changes of plasma NAD concentration were not mentioned, and the trial was an open-label single oral dosing with only 10 healthy male participants [9]. The first randomized controlled trial (RCT) of NMN supplementation revealed the increases of skeletal muscle insulin sensitivity and signaling, and significant elevation of NAD concentration in peripheral blood mononuclear cell (PBMC), but found no significant change in muscle NAD concentration, and the trial used only a fixed 250 mg/day oral dose with prediabetic and obese female participants [10]. Another RCT found that the NMN supplementation was safe, but the increase of blood NAD concentration was not statistically significant with 300 mg/day oral dose on 62 middle-aged healthy men and women for 60 days [11]. A similar RCT reported that oral 300 mg/day NMN supplement was safe and significantly improved several blood biomarkers, such as HbA1c and HDL-C, but has significantly reduced blood NAD level for the 17 postmenopausal female participants after an 8-week trial [12]. A 12-week RCT with a fixed 250 mg/day oral NMN dose on 42 healthy older men showed that blood NAD concentration was significantly elevated and NMN was well tolerated, but NMN supplementation produced mixed results on skeletal muscle strength tests [13]. A separate RCT disclosed that, after intervention also with a fixed 250 mg/day NMN oral dose for 12 weeks on 30 healthy males and females, blood NAD concentration increased significantly starting at week 4 until the end of treatment at week 12 and returned to original level 4 weeks after NMN treatment, and the NMN supplementation was safe and well tolerated [14]. An RCT with 1000 and 2000 mg/day oral doses of MIB-626, a unique microcrystalline NMN formulation, on 32 overweight or obese older adults showed that blood NAD concentration was dose-dependently increased and NMN treatment was also safe and well tolerated, but the trial found that lower doses failed to significantly elevate blood NAD concentrations [15]. A 6-week RCT with healthy amateur athletes with 300, 600, and 1200 mg/day NMN oral regimens concluded that exercise plus NMN supplementation was significantly and dose-dependently increased aerobic capacity but found no impact on physical strength when compared to exercise alone [16]. Another RCT on the effects of the time-dependent intake (morning or afternoon) of NMN on sleep quality, fatigue, and physical performance in older adults was conducted with 250 mg/day NMN oral treatment for 12 weeks [17]. The trial found that only drowsiness and lower limb function in the afternoon NMN-treated group were significantly improved among the many trial end points measured. In summary, NMN supplementation was found safe and well tolerated in all the above disclosed human clinical trials [9–17]. However, only mixed results were reported on the effects of NMN to human NAD elevation [9–15] and age-related physical decline and health conditions [9–13, 16, 17]. Most of these studies used either 250 or 300 mg/day fixed dosage [10–14, 17] or only recruited either male [9, 13] or female participants [10, 12] or participants with certain health conditions such as obese and overweight [10, 15]. Further clinical studies are needed to evaluate the effects of NMN supplementation on healthy middle-aged adults of both males and females with dose-dependent oral dosing regimens in the 300-900 mg/day range on the effects of NAD concentration, safety and tolerability, and age-related physical decline and health conditions.
![]()
| β-Nicotinamide Mononucleotide Basic information |
| Product Name: | β-Nicotinamide Mononucleotide |
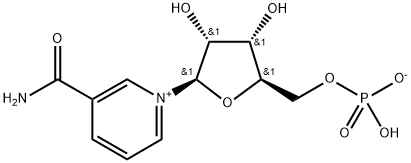
| Synonyms: | BETA-NMN;BETA-NICOTINAMIDE MONONUCLEOTIDE;BETA-NICOTINAMIDE RIBOSE MONOPHOSPHATE;3-(aminocarbonyl)-1-(5-O-phosphonato-beta-D-ribofuranosyl)pyridinium;B-NICOTINAMIDE MONONUCLEOTIDE;NMN;NICOTINAMIDE-1-IUM-1-BETA-D-RIBOFURANOSIDE 5'-PHOSPHATE;NICOTINAMIDE RIBOTIDE |
| CAS: | 1094-61-7 |
| MF: | C11H15N2O8P |
| MW: | 334.22 |
| EINECS: | 214-136-5 |
| Product Categories: | nicotinamide mononucleotide;Pharmaceuticals;Amines;Aromatics;Bases & Related Reagents;API;Intermediates & Fine Chemicals;Nicotine Derivatives;Nucleotides;BETA-NICOTINAMI |
| Mol File: | 1094-61-7.mol |
 |
|
| β-Nicotinamide Mononucleotide Chemical Properties |
| Melting point | 166 °C(dec.) |
| storage temp. | 2-8°C |
| Merck | 13,6697 |
| BRN | 3570187 |
| InChIKey | DAYLJWODMCOQEW-TURQNECASA-N |
| Safety Information |
| WGK Germany | 3 |
| F | 8-10-21 |
| MSDS Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| β-Nicotinamide Mononucleotide Usage And Synthesis |
| Chemical Properties | White to Yellowish lyophilized powder |
| Uses | A product of the extracellular Nicotinamide phosphoribosyltransferase (eNAMPT) reaction and a key NAD+ intermediate. It ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice . It also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. |
| Uses | β-Nicotinamide mononucleotide (NMN) is used to study binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments. Nicotinamide mononucleotide ("NMN" and "β-NMN") is a nucleotide derived from ribose and nicotinamide. Niacinamide (nicotinamide,) is a derivative of vitamin B3, also known as niacin.) As a biochemical precursor of NAD+, it may be useful in the prevention of pellagra. β-Nicotinamide mononucleotide (β-NMN) is an intermediate in the biosynthesis of nicotinamide adenine dinucleotide (NAD+). Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to generate β-NMN, which is subsequently converted to NAD+ by β-NMN adenyltransferase.At 50-100 µM, β-NMN has been used to enhance NAD biosynthesis and glucose-stimulated insulin secretion in a Nampt+/- mouse model of metabolic disease, demonstrating a role for Nampt in β cell function.Furthermore, at 500 mg/kg/day, it has been shown to ameliorate glucose intolerance in high-fat diet-induced type 2 diabetes mice by restoring NAD+ levels. |
| Preparation | β-Nicotinamide mononucleotide is a NAD+intermediate. In recent years, the relation of NAD+metabolism and aging-associated disease is attracting attention from various research fields. Synthesis of β-nicotinamide mononucleotide (NMN) A solution of NAD (3.5 g, 5.28 mmol) and ZrCl4(6.15 g, 26.4 mmol) in 500 ml water was stirred at 50°C for 30 min. The hydrolysis was monitored by TLC (SiO2EtOH/ 1 M NH4Ac [7 : 3]). The reaction was quenched with 245mL of a 0.5 M solution of Na3PO4. After adjusting to pH 7 with a 2 M solution of HCl, a white precipitate was formed. The suspension was centrifuged 8 min,1,000rpm, the supernatant was collected and the pellet was washed two times with 200 mL water. The combined supernatants wereconcentrated to 1/3 of its volume on a rotary evaporator. The remaining solution was purified with a column filled with Dowex 50WX8 (100-200 mesh, H+-Form, column-material: 2.5 x 30 cm). The column was loaded with 1.5 L5 % HCl and equilibrated with1.5L millipore water until pH 5 was reached. 100 mL of the concentrated solution was loaded on the ion exchange column and eluted with Milliporewater. The first cleavage product eluted was NMN (615 mg, 1.84 mmol,yield:35 %) and yielded a colorless solid after evaporation of the solvent, followed by AMP. 1H NMR (500MHz, D2O)δ: 9.48 (s, 1 H), 9.31 (d,J= 6.2 Hz, 1 H), 9.00 (d,J= 8.2 Hz, 1 H), 8.32 (dd,J= 8.2, 6.2 Hz, 1 H), 6.24 (d,J=5.4 Hz, 1 H), 4.68-4.64 (m, 1 H), 4.58 (t, 1 H), 4.48-4.45 (m, 1 H), 4.36–4.14 (m,J= 12.0, 2 H). 13C NMR (75 MHz, d2o) δ: 165.50, 145.65, 142.15, 139.53, 133.62, 128.19, 99.65, 87.18, 87.06, 77.42, 70.71, 63.89, 63.82. 31P NMR (202 MHz, D2O)δ:-0.03 |
| Definition | A condensation product of nicotinamide and ribose 5-phosphate, in which the nitrogen of nicotinamide is linked to the (β) c-l of the ribose. |
| Clinical Use | Nicotinamide adenine dinucleotide (NAD+)?exists in all living organisms and is well known as a coenzyme in oxidation?reduction reaction. Recent research has drawn attention to its relation to “sirtuin” represented by NAD+?dependent protein deacetylases, which is to regulate various life phenomena as aging, people are especially paying high attention to “Anti-aging effect”. The turnover of the oxidized form of nicotinamide adenine dinucleotide (NAD+) has attracted interest in regard to longevity.?Meanwhile, sirtuin is NAD+?dependent and increase of cellular NAD+?level is expected to have similar effects to the STACs, thus nicotinamide mononucleotide, an intermediate of NAD+?biosynthesis, is also in the spotlight. |
| Purification Methods | Purify NMN by passage through a column of Dowex-1 (Clform) and washing with H2O until no absorbance is observed at 260 nm. The tubes containing NMN are pooled, adjusted to pH 5.5-6 and evaporated in vacuo to a small volume. This is adjusted to pH 3 with dilute HNO3 in an ice-bath and treated with 20volumes of Me2CO at 0-5o. The heavy white precipitate is collected by centrifugation at 0o. It is best stored wet and frozen or it can be dried to give a gummy residue. It has max 266nm ( 4,600) and min 249nm ( 3600) at pH 7.0 (i.e. no absorption at 340nm). It can be estimated by reaction with CNor hydrosulfite which form the 4-adducts (equivalent to NADH) which have UV max 340nm ( 6,200). Thus after reaction, an OD340 of one is obtained from a 0.1612mM solution in a 1cm path cuvette. [Plaut & Plaut Biochemical Preparations 5 56 1957, Maplan & Stolzenbach Methods Enzymol 3 899 1957, Kaplan et al. J Am Chem Soc 77 815 1955, Beilstein 22/2 V 168.] |











